While hydroponics provides a mechanism for all kinds of trial and error experimentation, there are still some rules we must obey. We can afford to make certain mistakes in pursuit of a better harvest, but one thing we can’t afford to get wrong is pH. Like so many other aspects of growing, there is much more going with pH than there appears to be on the surface. Understanding the details of pH before you grab a pH meter and start pushing buttons is one of the keys to mastering our craft so this week we’ll give you a comprehensive look at the fundamentals of pH, as well as a great deal on the tools that will help you achieve pH perfection!
What is pH?
Simply put, pH is a measure of acidity or alkalinity, but there is much more to it than that as it pertains to hydroponics. Like many of us, you may have wondered why pH is written the way it is. The reason for this seemingly backward abbreviation – it’s based on the activity of hydrogen ions, and “H” is the atomic symbol for hydrogen (hence, always capitalized). Interestingly, you will hear pH referred to as both (the) “power of hydrogen” or “potential of hydrogen” because it turns out that the Danish chemist who introduced the concept met his end before actually spelling out the “p” itself. This led to some debate over whether it was intended to represent “power” (stemming from German/French) or “potential” (stemming from Latin) as either term would be fitting in this context. For the sake of being consistent, we’ll stick with “potential of hydrogen” for the rest of our post.
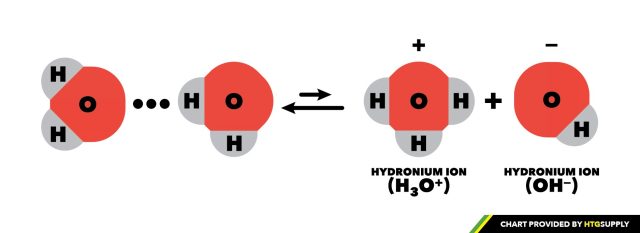
Potential of Hydrogen – So what exactly does “potential of hydrogen” (pH) mean? As we referenced above, pH indicates relative concentrations of ions in an aqueous solution, namely, hydronium (H3O+ or H+ for short) and hydroxide (OH-) in water (H2O). Water can be both acidic and alkaline, as well as pH neutral. Occasionally, a water molecule will lose one of its hydrogen atoms creating OH-. That hydrogen atom, in turn, latches onto another water molecule creating H3O+/H+. Pure water will have equal concentrations of both hydronium and hydroxide ions. The higher the concentration of H+, the more acidic the solution, while a higher concentration of OH will create alkalinity.
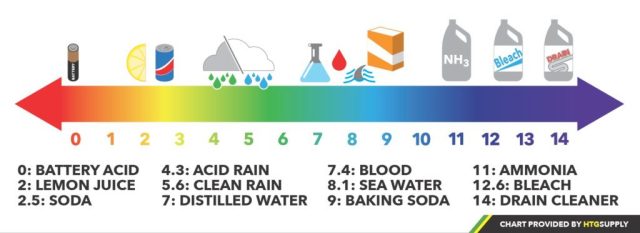
The pH Scale – The pH scale is represented using numbers 0-14, with 0 being very acidic substances such as battery acid, very alkaline substances like bleach near 14, and pure water right in the middle at 7 (neutral). It may seem like we’re only dealing with small changes when you look at these numbers, but remember – we’re talking about ions here, which are incredibly tiny particles! The pH scale is a shorthand way of expressing the negative logarithm (-log) of hydronium ions (H+) in a solution or (pH = -log [H+]). Logarithms or logs for short, are used to express large numbers in science and math. The pH scale uses a base 10 logarithm, which means each number represents a 10^2 change in H+ (or OH-) concentration. In more basic terms, pH raises 10-fold for every whole unit change, and this means we’re dealing with significant changes in acidity/alkalinity within small windows of the scale. For example, a pH of 6 has ten times the concentration of hydronium ions than a pH of 7, and a pH of 8 has ten that of a pH of 7. Following this further, a solution with a pH of 9 has one hundred times the concentration of hydroxide ions as a solution with a pH of 7.
Why is pH Important for Hydroponics?
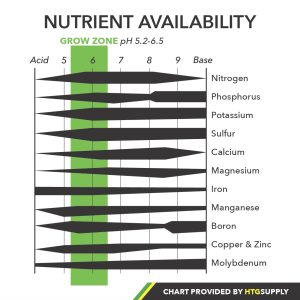
To be clear, pH is just as important for soil gardening as it is for hydroponics, but managing soil pH is almost an entirely different discipline so for the sake of all, we’ve chosen to focus on pH as it pertains to hydroponics in this article. In either case, the importance of pH basically boils down to nutrient availability. In order for plants to grow and produce in a hydroponic system, the mineral elements they need must be fully dissolved into the nutrient solution. So when we’re referring to nutrients as being available in hydroponics, we simply mean that they are fully dissolved into the solution. In order for minerals to dissolve into a nutrient solution, their molecules must be able to match or overcome the attractive forces of the water molecules themselves, and the ability of nutrients to dissolve or remain dissolved is directly affected by pH. If pH becomes too high or too low, it will cause minerals to become locked out or precipitate out of the solution, which means that they are no longer in a dissolved state.
One of the trickier parts in all of this is that minerals all have a different range in which they remain dissolved in a solution. For example, elements such as iron, manganese boron, copper, and zinc are most available at 5.5pH-5.8pH, whereas nitrogen, potassium, and phosphorus are most available from 6.0ph-6.5pH. These nutrients are still available outside of their optimal ranges, but they can become locked out altogether if pH swings too far one way or the other. What we’re shooting for in maintaining pH is a range in which the highest percentage of minerals are available across the board. The larger pH window we have to work with is generally accepted as 5.2-6.5, while the more optimal range in terms of broad spectrum availability is 5.8-6.2. These ranges fall within what is referred to as the “Grow Zone pH”. Anything over 6.5 and nutrients will begin to precipitate out of the solution. An example of this would be the behavior of iron. At a pH of about 7.3, only half of the iron ions will remain, and at a pH of 8, all of the iron will have been fully precipitated. In addition to the general range, most nutrient companies specify the ideal pH for their products, which should be observed for the best results.
Nutrient Solution pH Influences
There are several factors that can affect the pH of a nutrient solution or cause it to ‘drift’ over time, and it actually happens that the interaction between plants and nutrients is the main driving force most of the time. Nutrient solutions are made up of both positively charged ions known as cations and negatively charged ions called anions, however, plants require electrical balance in order to take in and utilize these nutrients. Because of this, plants will generate H+, a positively charged ion to help facilitate transport and processing when they experience incoming anions. As a byproduct of the plant’s own chemical processes, the extra H+ bonds with oxygen and is released back into the solution as OH-, which causes pH to rise. The opposite occurs when plants absorb cations or positively charged particles. Some minerals such as nitrogen can be supplied to plants in both forms to help offset this effect, but this is not the case with all nutrients so pH will change over time as more nutrients are used. This is important to remember because rapid changes in pH can indicate the plants are feeding at a high rate. If you’re experiencing substantial pH increases within a 24-48 hour period, this could indicate that the mineral supply in the solution is significantly depleted, and the solution should be changed out at this point for a fresh batch. In this case, adjusting pH is only masking the cause of the issue.

Another major pH influencer is water quality or the presence of elevated mineral levels in the source water. Both municipal water sources and well supplies can have a high alkalinity due to mineral and chemical content, but wells are particularly prone to this issue in the form of ‘hard water’. Alkaline, or high pH source water will offer less pH stability and require a far greater amount of acid (pH down) to make adjustments. This not only complicates monitoring but also sometimes leads to imbalances in the actual nutrient composition of the solution. If you’re regularly adding large amounts of pH down to your nutrient solution in order to maintain a proper pH, chances are you have alkalinity issues. In this case, filtration using a reverse osmosis system may be necessary. Daily monitoring is still advised, even when using an RO system, but starting out with pH neutral water should mean that little adjusting will be required until it’s actually about time to add more nutrients.
Other contributors to pH fluctuation include grow mediums, growth phase, and even the particular growing method in use. Systems with large, vigorously feeding plants and lower volumes of solution can experience more rapid pH changes. And while grow mediums can affect pH due to the natural acidity/alkalinity of the medium itself or the buildup of salts in the medium over time, the effects of growth phase on pH are a bit more abstract. As plants progress through the grow cycle, they will absorb different nutrients at different rates. This not only means that pH will change differently throughout the grow cycle, but also indicates that there will be slight differences in the optimal pH range for each part of the grow cycle. Many growers will prefer a pH of 5.8-6.0 during the vegetative stage for well-rounded availability, and a slightly higher pH of 6.0-6.2 during bloom for increased potassium and phosphorus uptake. It should be also be noted that different plants have varying pH preferences, even in a hydroponic setting, so doing your homework on what you’re growing in regard to pH is recommended.
Testing pH

There are many options for testing including simple, low-cost options such as pH strips or liquid pH test kits. Liquid or chemical tests typically involve filling a small vial with solution and adding a few drops of the pH-sensitive test dye. The pH is then obtained based on the color of the solution. Similarly, test strips or pH papers are simply dipped into a sample of the nutrient solution and matched to a color on the pH scale. While these testing methods are simple and cost-effective, they are not the most accurate means of testing. Digital pH meters are the preferred means of taking precision readings, but using a meter isn’t as simple as turning it on and dipping it in the solution. Meters are sensitive tools, and like any other precision instrument, they must be cared for and maintained themselves. Neglected or misused meters will provide incorrect results because of contamination or probe deterioration. Electrodes will need to be replaced at certain intervals even with proper use and care, but you will ensure accurate readings and maximize the lifespan of your meter’s electrode by following these best practices:
Rinse the probe in distilled or RO water before and after each use
Calibrate the meter every 20-30 uses
Clean the meter with a soft-bristled brush when calibrating or if the probe is visibly dirty
Never store in water, use the correct storage solution
Never touch the probe directly with hands
Adjusting pH
When it comes to adjusting pH, the process is pretty straightforward and usually accomplished through the use pH up and down formulas. While there are plenty different substances that could be used to adjust pH including household items like lemon juice, these options typically only offer short-term balancing and may also introduce compounds into the solution that contribute to the growth of pathogens. Therefore, we recommend sticking with the stuff that’s formulated by the pros. One important side note about adjusting pH – adjusters should always be diluted before being added to a nutrient solution. Remember when we talked about nutrient lockout above? Adding pH up or pH down directly into the solution can create localized extremes in pH, which will cause nutrients to bind and precipitate out of your solution. Aside from helping to prevent this issue, diluting pH down/up in a few gallons of water before use will also help you make finer adjustments and avoid using excess amounts of adjuster. From there, simply add small amounts of the diluted pH adjuster solution until you reach the proper level.
Maintaining Grow Zone pH
Understanding the fundamentals of pH and the underlying forces at work gives you the upper hand when it comes to maintaining optimal pH for growth, and keeping a good pH balance requires a daily regimen of both testing and adjusting. It may all seem a bit tedious, but with so many variables at hand, it is an absolute must unless you’re prepared to fully automate the process. Even if you do, keeping a close eye on the system is certainly wise. Whatever route you decide to take, HTG Supply has the tools and supplies you need to employ perfect pH practices!
______________
Questions, comments, or tips of your own? Feel free to join the conversation – we’d love to hear from you! And don’t forget to check out this week’s coupon code and sale information below! From all of us here, good luck, stay safe, and Happy Growing!

THIS WEEK’S COUPON CODE: PH122217
Enter this week’s promo code at checkout for a 10% discount on any pH meter in stock or visit your local store and simply mention this article to get the deal! Thanks again for tuning into Talking Shop with HTG Supply! Offer valid through HTGSupply.com and in-store 12/22/17-12/29/17. Cannot be combined with other offers. Follow us on social media for all the Sales, Events and Customer Appreciation Days. In addition, learn more about indoor growing and get all kinds of tips, tricks, and techniques!


